
Safety of NATPARin hypoparathyroidism
NATPAR was well tolerated in patients with chronic hypoparathyroidism1
In clinical studies, adverse reactions were generally mild to moderate in severity and transient, and they were managed with adjustments of NATPAR, calcium and/or active vitamin D doses.1
List of adverse reactions associated with NATPAR in patients with chronic hypoparathyroidism1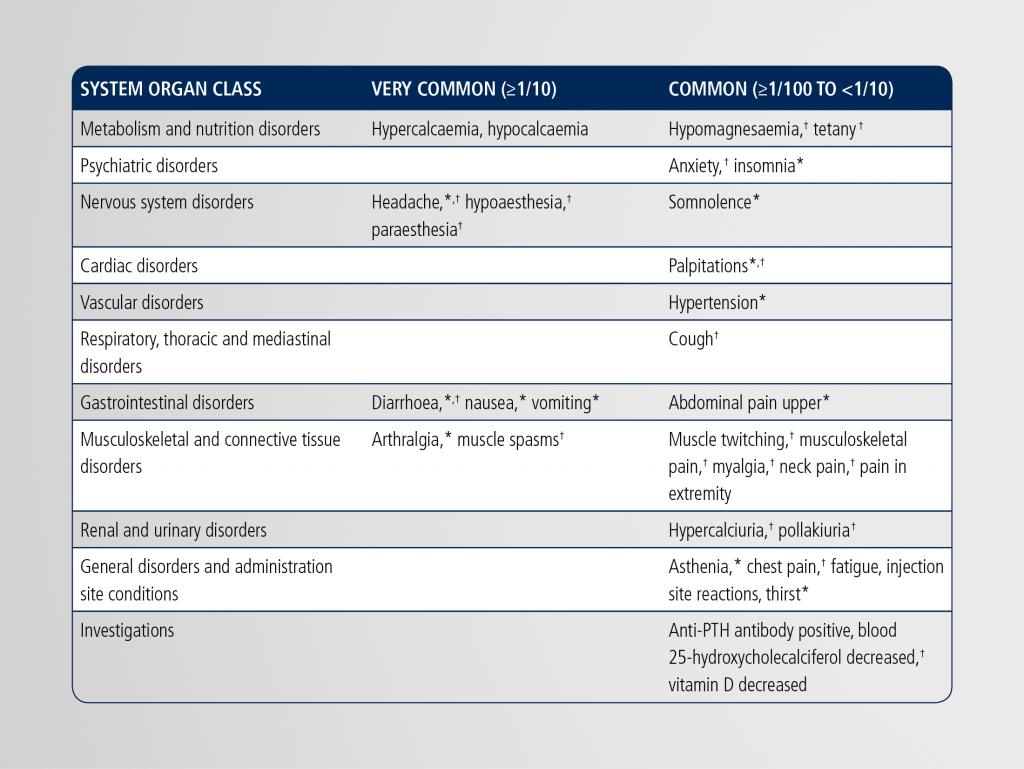
PTH, parathyroid hormone
*Signs and symptoms potentially associated with hypercalcaemia that were observed in the clinical trials.
†Signs and symptoms potentially associated with hypocalcaemia that were observed in the clinical trials.
Long-term safety of NATPAR was demonstrated at 60 months1,2
In addition to the 60-month results from the RACE study,1,2 a 72-month study showed that NATPAR was well-tolerated as a long-term treatment for chronic hypoparathyroidism.3
LONG-TERM STUDY: Prospective 72-month open-label study to evaluate the long-term safety and efficacy of NATPAR for the treatment of chronic hypoparathyroidism on biochemical, densitometric and dynamic skeletal indices (n=33). Main inclusion criterion was hypoparathyroidism for ≥12 months. Initial dose was 100 μg with possible titration to 25, 50 or 75 μg. The main goal was to reach a target dose of 1.5 g/day of calcium and 0.25 μg/day of 1,25-dihydroxyvitamin D and not to remove calcitriol altogether.3
References
- NATPAR Summary of Product Characteristics.
- Mannstadt M, Clarke BL, Bilezikian JP, et al. Safety and Efficacy of 5 Years of Treatment With Recombinant Human Parathyroid Hormone in Adults With Hypoparathyroidism. J Clin Endocrinol Metab. 2019;104(11):5136-47.
- Rubin MR, Cusano NE, Fan WW, et al. Therapy of hypoparathyroidism with PTH(1-84): a prospective six year investigation of efficacy and safety. J Clin Endocrinol Metab 2016;101(7):2742–50.
Natpar® (parathyroid hormone (rDNA)
Detailed Safety Information
Please consult the Natpar Summary Product Characteristics (SmPC) before prescribing.
Natpar treatment should be supervised by a physician or other qualified healthcare professional experienced in the management of patients with hypoparathyroidism. The goal of treatment is to achieve calcaemic control and to reduce symptoms. The optimisation of parameters of calcium phosphate metabolism should be in line with current therapeutic guidelines for the treatment of hypoparathyroidism. Prior to initiating and during treatment with Natpar confirm that 25-OH vitamin D stores are sufficient and that serum magnesium is within the reference range.
Contraindications
Natpar is contraindicated in patients with hypersensitivity to the active substance or to any of the excipients, who are receiving or who have previously received radiation therapy to the skeleton, with skeletal malignancies or bone metastases, who are at increased baseline risk for osteosarcoma, with unexplained elevations of bone-specific alkaline phosphatase, with pseudohypoparathyroidism.
Warnings and Precautions
Monitoring of patients during treatment: pre-dose and in some cases post-dose serum calcium levels must be monitored during treatment with Natpar.
Hypercalcaemia: this was reported in clinical trials with Natpar. Hypercalcaemia commonly occurred during the titration period, during which doses of oral calcium, active vitamin D, and Natpar were being adjusted. Hypercalcaemia may be minimized by following the recommended dosing, the monitoring information and asking patients about any symptoms of hypercalcaemia. If severe hypercalcaemia develops, hydration and temporarily stopping Natpar, calcium and active vitamin D should be considered until serum calcium returns to the normal range. Then consider resuming Natpar, calcium and active vitamin D at lower doses.
Hypocalcaemia: a common clinical manifestation of hypoparathyroidism was reported in clinical trials with Natpar. Most of the hypocalcaemic events occurring in the clinical trials were mild to moderate severity. The risk for serious hypocalcaemia was greatest after the withdrawal of Natpar. Temporary or permanent discontinuation of Natpar must be accompanied by monitoring of serum calcium levels and increase of exogenous calcium and/or vitamin D sources as necessary. Hypocalcaemia may be minimized by following the recommended dosing, the monitoring information, and asking patients about any symptoms of hypocalcaemia.
Concomitant use with cardiac glycosides: Hypercalcaemia of any cause may predispose to digitalis toxicity, monitor serum calcium and cardiac glycoside levels and patients for signs and symptoms of digitalis toxicity.
Severe renal or hepatic disease: Natpar should be used with caution in patients with severe renal or hepatic disease because they have not been evaluated in clinical trials.
Use in young adults: Natpar should be used with caution in young adult patients with open epiphyses.
Tachyphylaxis: the calcium-raising effect of Natpar may diminish over time in some patients. The response of serum calcium concentration to administration of Natpar should be monitored at intervals to detect this and the diagnosis of tachyphylaxis considered.
Urolithiasis: Natpar has not been studied in patients with urolithiasis. Natpar should be used with caution in patients with active or recent urolithiasis because of the potential to exacerbate this condition.
Adverse Reactions
The most commonly observed adverse events with Natpar treatment were hypercalcaemia, hypocalcaemia, headache, diarrhoea, vomiting, paraesthesia, hypoaesthesia and hypercalciuria.
|
Very common
(frequency ≥1/10): |
Hypercalcaemia, hypocalcaemia, headache, hypoaesthesia, paraesthesia, diarrhoea, nausea, vomiting, arthralgia, and muscle spasms. |
|
Common
(≥1/100 to <1/10): |
Hypomagnesaemia, tetany, anxiety, insomnia, somnolence, palpitations, hypertension, cough, upper abdominal pain, muscle twitching, musculoskeletal pain, myalgia, neck pain, pain in extremities, hypercalciuria, pollakiuria, asthenia, chest pain, fatigue, injection site reactions, thirst, anti-PTH antibody positive, blood 25-hydroxycholecalciferol decreased, vitamin D decreased. |
Material code: pi-00658
Date of Preparation of SI: February 2019
Prescribing Information
| Body weight (kg) | Volume to be injected (mL) |
|---|---|
| 38–41 | 0.xx |
| 42–45 | 0.xx |
| 46–49 | 0.xx |
| 50–53 | 0.xx |
| 54–57 | 0.xx |
| 58–61 | 0.xx |
| 62–65 | 0.xx |
| 66–69 | 0.xx |
| 70–73 | 0.xx |
| 74–77 | 0.xx |
| 78–81 | 0.xx |
| 82–85 | 0.xx |
| 86–89 | 0.xx |
| 90–93 | 0.xx |